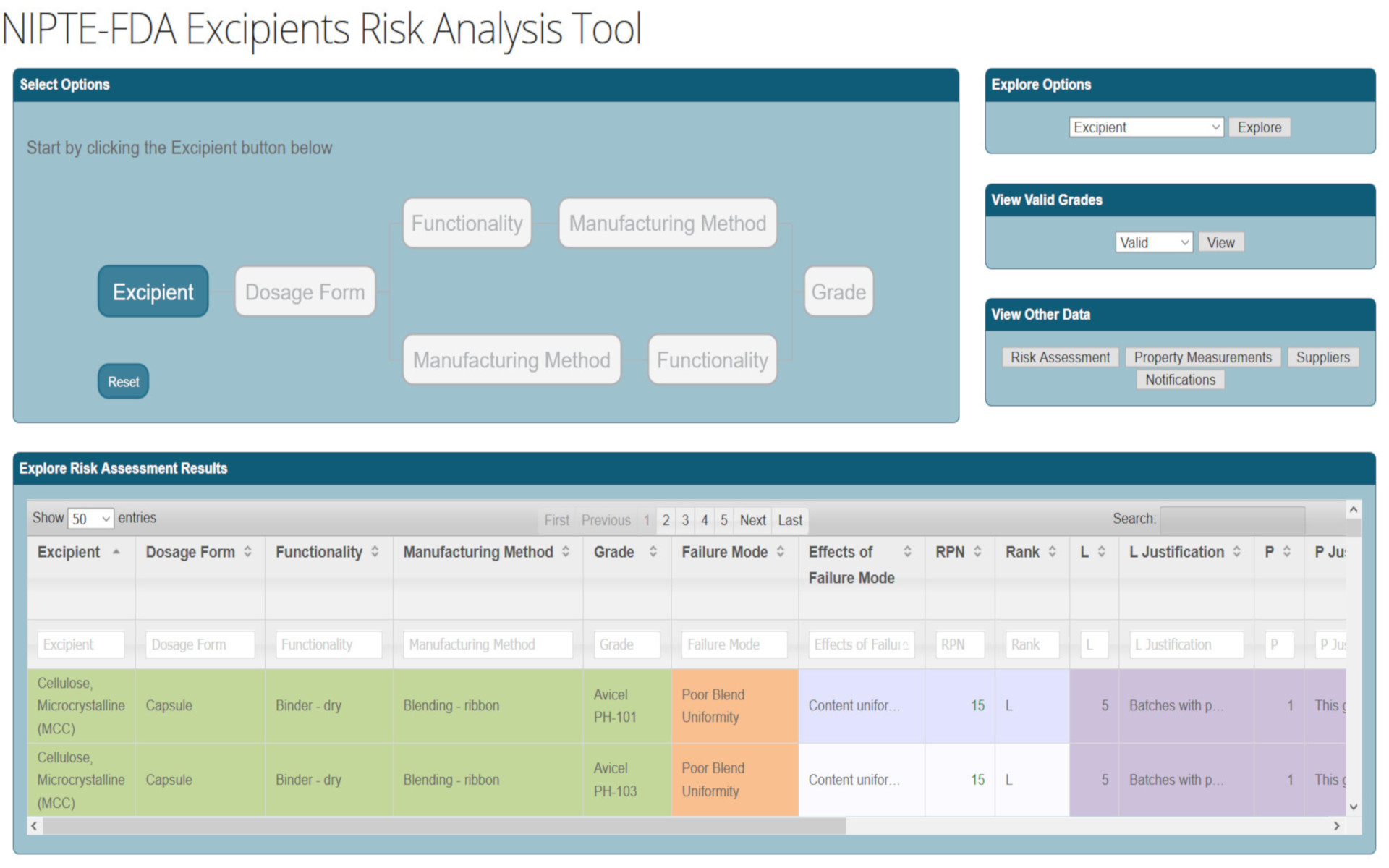
From the web page: Excipients play a critical role in the manufacturability and clinical performance of dosage forms. To evaluate the risks associated with excipient selection, characterization, and use, we developed a risk analysis decision support tool. The tool couples risk assessment and risk narratives with catalogs of excipients, dosage form types, functionalities, and manufacturing methods. Our tool can be used by industry during development to provide sound integrated risk analysis that addresses questions and concerns about stability, drug delivery performance, direct and indirect effects related to impurities and toxicity, and other issues. It can also be used by the FDA for integrated NDA reviews and efficient knowledge transfer to CGMP inspectors on risk factors to consider during their approval processes for product quality and safety. My contribution on this project is the development of the risk assessment tool for a manufacturing path (link here). The tool was developed using PHP, SVG (for UI) and jQuery. The data visualization is performed using the dataviewer component from HubZero.